At OLIGOFASTX we are working to achieve solutions that improve the quality of life of patients affected by rare diseases, and for this we use all the power of technology, knowledge and the expertise from our partners. Like any other public or private entity, all our developments are subject to the different drug authorization processes operating in Spain, procedures that are different to a greater or lesser extent depending on the country in question;
This means we have a long way to go until our goal becomes a reality;
The Spanish Agency of Medicines and Health Products (AEMPS) is the regulatory body that ensures strict compliance with each step of the process, with the aim of ensuring that any authorization is based on objective scientific facts and data, thus safeguarding the health of the population;
According to the latest published report on activities carried out by the AEMPS in 2021, the number of drugs authorized for distribution and marketing was 1,161; Likewise, 996 clinical trials were also authorized;
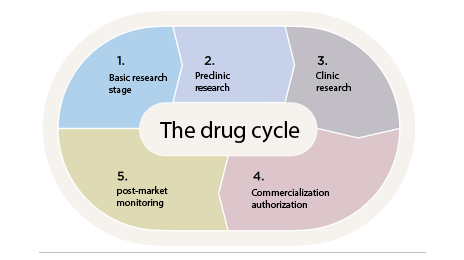
The authorization procedure for a drug in Spain
Medicines are regulated throughout their life cycle; All drugs used in Spain must have a marketing authorization granted by the AEMPS once their quality, safety and efficacy have been favorably evaluated, and any variation must also be authorized or notified to the AEMPS;
These evaluations ensure that a positive benefit-risk ratio is maintained throughout the life cycle of the drug on the market;
Source: https://www.aemps.gob.es/publicaciones/publica/regulacion_med-PS/v2/docs/reg_med-PS-v2-light.pdf
Submission of a marketing authorization dossier
No drug may be marketed in Spain without prior authorization from the European Commission following the recommendation of the EMA;
Marketing authorizationis granted on the basis of scientific criteria on the quality, safety and efficacy of the medicinal product concerned. These three criteria make it possible to evaluate the relationship between the benefits and risks of the drug for the diseases and situations for which it is approved;
- For years, there have been common technical criteria in the European Union (EU) for the evaluation and authorization of medicinal products;
After its authorization, the drug is subject to constant monitoring of developments in terms of risks and new uses, so that at any time such authorization may be reviewed.
Any changes to a drug once authorized must be evaluated following the same procedure as its original authorization.
Pharmaceutical companies must therefore decide at an early stage of development what type of application to submit for marketing authorization;
Special pediatric process
Specific regulations are in place to facilitate the development and accessibility of medicines for pediatric use, to ensure that such medicines are the result of ethical and quality research and are specifically authorized for use in the pediatric population, and to improve the information available on the use of medicines in different pediatric populations;
The procedure will be initiated by the marketing authorization holders by e-mail to the mailbox created for this purpose by the AEMPS; More information about this procedure here.
Authorization procedures
There are four different authorization procedures:
- National procedure. The applicant submits to the AEMPS the dossier with all the information for the marketing authorization of the drug in Spain.
- Decentralized procedure. The applicant submits its application for authorization simultaneously in several countries of the European Union.
- Mutual recognition procedure. It is used when a drug already has a Community marketing authorization.
- Centralized procedure. The applicant applies for an authorization for all EU Member States at the same time.
Abbreviated revalidation
The Spanish Agency of Medicines and Health Products (AEMPS) has modified the procedure for revalidation of the marketing authorization. As of March 1, 2009, an abbreviated revalidation is now required, regardless of its legal basis. This change is carried out in accordance with the new criteria established by the EMA through the Decentralized Procedures and Mutual Recognition Coordination Group (CMDh) of the Heads of Medicines Agency (HMA) Network and which are included in the guide CMDh Best Practice Guide on the processing of renewals in the Mutual Recognition and Decentralised Procedures, published past february.
With the intention of simplifying the regulatory process, the CMDh has agreed to apply the same criteria to all medicines for human use, regardless of their legal basis; This criterion is the one already used for generic drugs applied for in accordance with Art. 10.1 of Directive 2001/83/EC: administrative revalidation. With this procedure only one cover letter, an electronic form (eAF) without attachments and proof of payment of the fee, with a 30-day schedule.
Despite these changes, Member States may continue to request additional documentation for national legislative reasons or, in exceptional cases and for reasons related to the benefit/risk balance of the medicinal product, may request a full renewal. In the latter case, the complete required documentation shall be submitted and the timetable shall be 90 days.
Additionally, since last June 28, 2023, the Law 38/2022, of December 27, which introduces changes in the application of AEMPS fees on medicines, medical devices, cosmetic products, personal care products, pharmaceutical laboratories and distribution entities.
Affordable, accessible and safe medicines for all: the European Commission launched a Pharmaceutical Strategy for Europe
The European Commission has recently launched a Pharmaceutical Strategy for Europe to ensure that patients have access to innovative and affordable medicines, as well as to promote the competitiveness, innovative capacity and sustainability of the EU pharmaceutical industry. This strategy will enable Europe to meet its pharmaceutical needs, even in times of crisis, through strong supply chains.
The European Pharmaceutical Strategy has four main objectives:
- ensure patient access to affordable drugs and address unmet medical needs (e.g., in the areas of antimicrobial resistance, cancer and rare diseases);
- foster the competitiveness, innovation and sustainability of the EU pharmaceutical industry and the development of high-quality, safe, effective and greener medicines;
- improve mechanisms for crisis preparedness and response and address security of supply;
- ensure a strong voice of the EU in the world promoting high standards of quality, efficacy and safety.
PRIME Framework: guaranteeing faster access to medicines
The European Medicines Agency (EMA) has launched PRIME (PRIority MEdicines – priority medicines), a scheme aimed at strengthening support for medicines that address an unmet medical need. Europe thus promises faster access to new drugs that are showing potential.
The scheme focuses on drugs that may offer a significant therapeutic advantage over existing treatments, or benefit patients with no treatment options. These drugs are considered priority drugs within the European Union (EU).
Through PRIME, the EMA offers proactive and enhanced support for drug developers to optimize the generation of reliable data on the benefits and risks of a drug and enable accelerated evaluation of drug applications. This will help patients benefit as quickly as possible from therapies that can significantly improve your quality of life.
Therapeutic Positioning Reports in Spain
The Spanish Agency of Medicines and Health Products (AEMPS) and the General Directorate of Basic Portfolio of the National Health Service and Pharmacy (DGCBSF) must evaluate medicines for human use for their effective incorporation into healthcare practice, each within the scope of their competencies. To avoid redundancies, since 2013 a single therapeutic positioning report has been prepared that is recognizable for the entire SNS through a networked evaluation system based on scientific evidence. In this way, the same final benefit is achieved for each of them, maintaining consistency in the evaluation and sharing resources more efficiently.
You can access all the official reports published here.
However, the regulation is under judicial review and is likely to change in the coming years.
Data in Spain in 2022
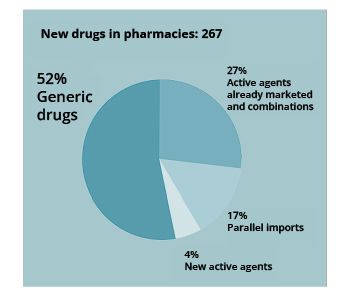
According to Farmaindustria, in the pharmacy office channel, 267 new drugs were marketed in 2022, of which 139 (52%) are generic drugs, 46 (17%) are parallel imports and 11 (4%) are drugs with new active ingredients.
The remaining 71 (27%) are drugs with active ingredients or combinations already on the market.
Source: https://www.farmaindustria.es/web/wp-content/uploads/sites/2/2023/07/farmaindustria-memoria-2022.pdf
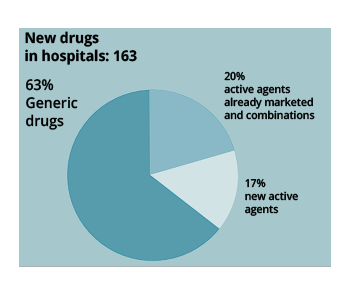
For its part, 163 new drugs were marketed in the hospital sector in 2022, of which 102 (63%) are generic drugs, 28 (17%) are drugs with new active ingredients, including 7 orphan drugs. The remaining 33 (20%) are drugs with existing active ingredients or combinations.
Source: https://www.farmaindustria.es/web/wp-content/uploads/sites/2/2023/07/farmaindustria-memoria-2022.pdf
To find out whether a drug is authorized or not, you can consult the AEMPS Online Drug Information Center (CIMA), available on the Internet at www.aemps.gob.es, which offers permanently updated information on all drugs authorized by the AEMPS.
According to the online Drug Information Center, there are currently 15,498 authorized drugs, 2,608 different active ingredients, 33,080 presentations, 385 biosimilars and 275 orphan drugs.
These categories and other specific procedures for bioconjugates, among others, will be discussed in future posts. In the meantime, you can follow us through social networks and keep up to date with everything we are doing at OLIGOFASTX.
Sources of information:
https://www.aemps.gob.es/publicaciones/publica/regulacion_med-PS/v2/docs/reg_med-PS-v2-light.pdf
https://www.farmaindustria.es/web/memoria-anual-2022/
https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0213-91112020000200008
https://www.aemps.gob.es/comunicacion/notas-informativas-medicamentos-de-uso-humano/?fec=2022&cat=49
https://cima.aemps.es/cima/publico/home.html#
https://www.aemps.gob.es/medicamentos-de-uso-humano/informes-de-posicionamiento-terapeutico/
https://ec.europa.eu/commission/presscorner/detail/es/ip_20_2173
https://toolbox.eupati.eu/resources/solicitudes-de-autorizacion-de-comercializacion/?lang=es

 Español
Español