In this fourth installment of our #DiscoveringOligonucleotides series, we explore a crucial challenge for innovative drug development, including those based on therapeutic oligonucleotides: delivery systems.
Challenges in the release of therapeutic oligonucleotides
Therapeutic oligonucleotides have enormous potential to address a variety of diseases, from genetic disorders to neurodegenerative diseases. However, their efficacy is often linked to their ability to reach target cells in sufficient quantities. This is where the importance of delivery systems comes in.
- The human body has natural defences (ribonucleases) that can degrade oligonucleotides before they reach their destination.
- In addition, these compounds often face biological barriers, such as cell membranes, which make it difficult for them to reach cells.
Delivery systems seek to overcome these challenges by ensuring that oligonucleotides reach their target effectively.
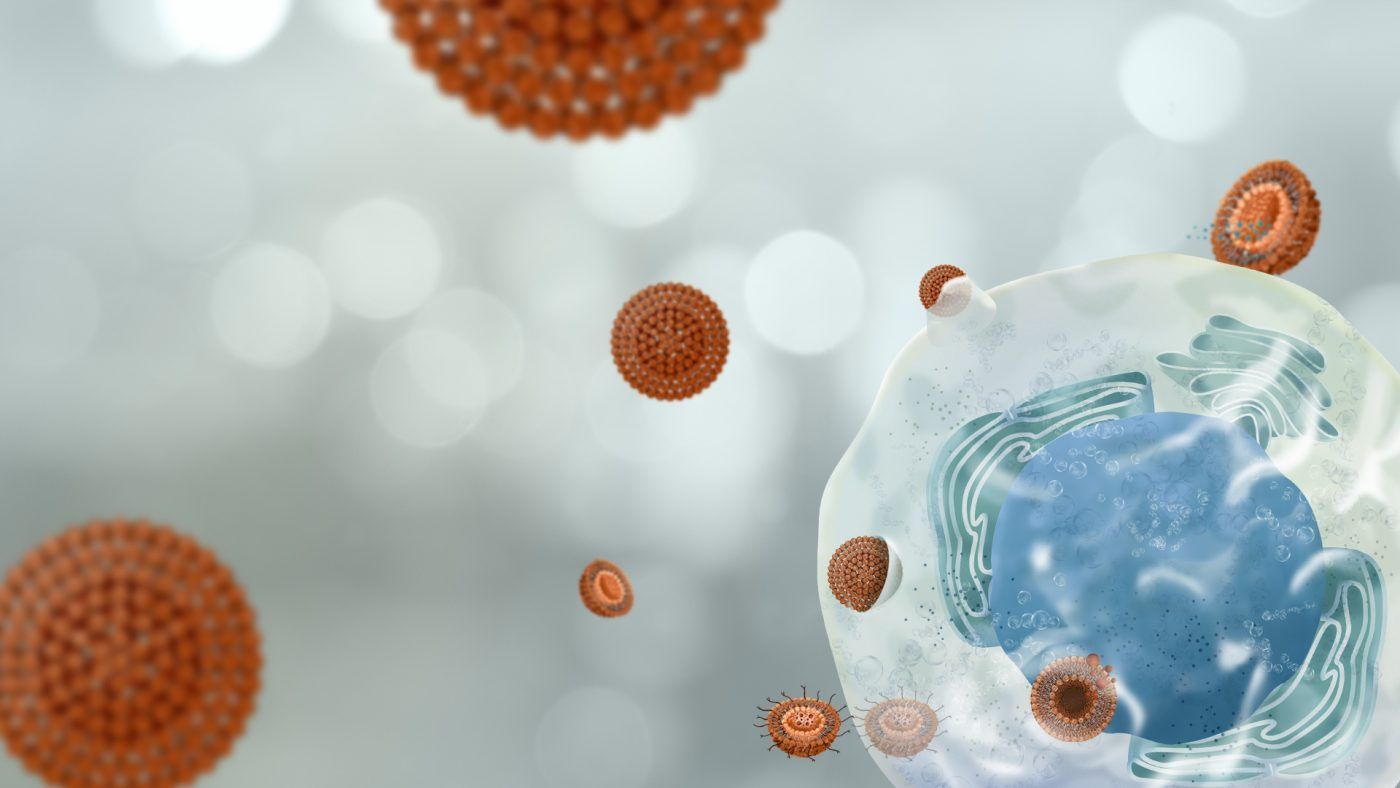
Types of release systems
To achieve the expected therapeutic effect, the most commonly used delivery systems for oligonucleotides are the application of nanotechnology (delivery) and bioconjugation.
- Delivery
The incorporation of a drug into a delivery system improves its solubility, protects it from harmful external agents such as oxygen and allows for targeted and controlled releases.
The vehiculization of a drug also enables better control of its pharmacokinetics, modification of the circulation time, and avoiding non-specific interactions thanks to an appropriate coating. This also makes other routes of administration possible due to increased solubility and protection against gastrointestinal disorders. Reaching difficult targets will be easier, making it possible to overcome the blood-brain barrier to treat or diagnose diseases of the central nervous system.
In addition, through a specific strategy, the nanocarrier can be tracked with non-invasive imaging techniques to learn its ADME profile or to detect disease biomarkers as a diagnostic method. Finally, and most importantly, the nano-vehicle will accumulate at the active site reducing toxicity issues.
The most developed strategies are:
- Lipid nanoparticles.Lipid nanoparticles are promising vehicles for the delivery of oligonucleotides. Composed of lipids, these particles can encapsulate and protect the oligonucleotides, improving their stability and allowing their controlled release at the right place.
- Polymeric nanoparticles. Polymers also play a crucial role in the design of release systems. Polymeric nanoparticles can be designed to release oligonucleotides in a sustained manner, thereby improving the bioavailability and efficacy of the treatment.
- Lipid-polymeric nanoparticles. The combination of lipids and polymers allows the formation of a versatile structure that can load and release drugs in a controlled manner to improve the efficacy of treatments and reduce adverse effects.
- Bioconjugation
Bioconjugates are currently one of the most widely used and promising strategies to deliver oligonucleotides into specific cells and organs and improve their properties.
Bioconjugation is a chemical strategy to form a stable covalent bond between two molecules, at least one of which is a biomolecule. This strategy allows the design of molecules with a dual function.
An active molecule with a therapeutic target – in this case, an oligonucleotide – binds with a strong chemical bond (covalent bond) to another functional molecule (it can be a protein, an oligonucleotide or any other structure) that can recognise specific molecules in the membrane of the cells of a target organ. In addition, this second molecule can also enhance the specific properties of the first molecules.
Inside the organism, once the functional molecule reaches the cells, the covalent bond is broken and the two molecules separate. The oligonucleotide-based therapeutic molecule will initiate the biological function for which it was designed.
Bioconjugation is based on a comprehensible and rational design of the molecules. Separately, the molecules that make up a bioconjugate have previously been well tested in the laboratory and their properties have been studied in depth. However, when joined together, the bioconjugated molecule has its own properties. Bioconjugated units are joined together by a particular unit called a linker. These linkers break under specific conditions. For example, they can be broken by enzymes or by the specific acidic environment inside the cells.
Since they are to be considered as new molecules and different from their units, bioconjugates have to go through the same pharmaceutical research and development process as any other new experimental drug.
- Possible linker molecules
Aptamers, antibodies, fatty acids and peptides are molecules that play a crucial role in the engineering of biomedical systems.
- Aptamers, which are single-stranded nucleic acid sequences selected for their high affinity for specific targets, offer unique versatility in molecular recognition.
- Antibodies are specialised proteins that recognise and bind to specific antigens, facilitating targeted drug delivery.
- Fatty acids, like lipids, act as efficient transporters and stabilisers in drug delivery systems.
- Finally, peptides, composed of short chains of amino acids, can mediate specific interactions, facilitating binding to therapeutic targets.
These linker molecules offer innovative strategies to improve the selectivity and efficacy of biomedical treatments, opening up new possibilities in personalised medicine and targeted therapy.
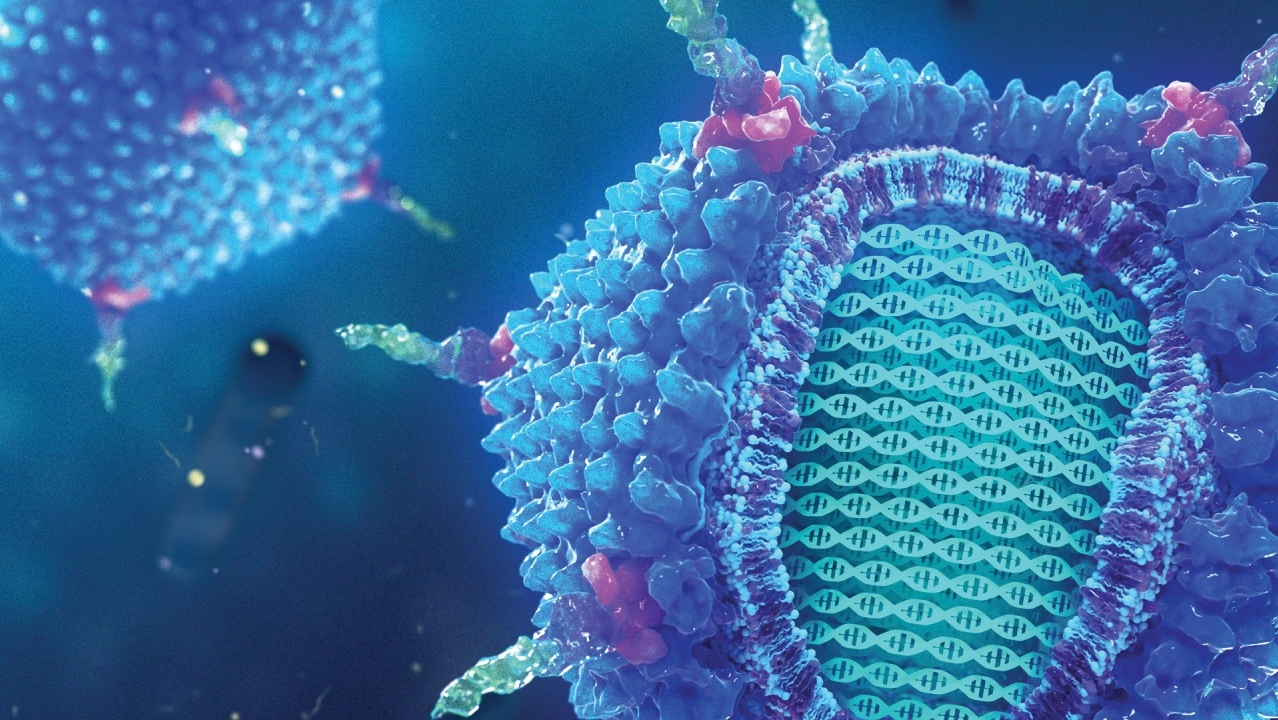
Controlled release
The key to the success of therapeutic oligonucleotides lies in controlled release. Systems designed to gradually release oligonucleotides at the desired site offer significant benefits, minimising side effects and maximising therapeutic efficacy.
The importance of controlled release becomes exceptionally relevant when addressing rare or undiagnosed diseases. In many cases, these conditions present unique and complex challenges, often stemming from specific genetic mutations. The ability to design delivery systems that deliver therapeutic oligonucleotides precisely to the affected cells becomes a determining factor for successful treatment.
These systems allow for highly personalised care, tailored to the individual genetic characteristics of patients, offering the hope of addressing conditions for which conventional therapies may not be effective. Targeted release not only maximises therapeutic efficacy, but also minimises potential side effects, offering a safer and more targeted approach to the treatment of rare and undiagnosed diseases.
Through a combination of cutting-edge technologies and personalised approaches, at OLIGOFASTX we work to deliver revolutionary treatments that bring hope and improve the quality of life for those facing exceptional and often overlooked medical conditions.
The future of delivery systems in therapeutic oligonucleotides
As research progresses, the scientific community is embarking on a quest for ever more precise and personalised delivery systems. Nanotechnology, tissue engineering and artificial intelligence are being integrated to design systems that not only deliver oligonucleotides efficiently, but also respond dynamically to the specific needs of each patient.
In short, therapeutic oligonucleotide delivery systems represent an essential piece in the puzzle of personalised and precision medicine. With each breakthrough, we are approaching a future where these small molecular messengers will unlock new frontiers in disease treatment, offering hope to those seeking innovative and effective solutions.
Sources:
Lipid nanoparticles: past, present and future opportunities | CAS
Nanomaterials for Therapeutic RNA Delivery – ScienceDirect
NEW MODIFIED RELEASE PHARMACEUTICAL FORMS: REVIEW AND RELEVANCE

 Español
Español
