The last few years have been a true revolution in terms of discoveries and initiatives linked to the world of oligonucleotides, and all eyes and hopes continue to focus in the same direction in the new year 2023.
It is clear that the research being carried out in the scientific world is yielding results. In this article we present new advances for different pathologies.
Familial amyotrophic lateral scleriosis
Researchers at the Scripps Institution have developed a molecule with potential for the treatment of genetically based amyotrophic lateral sclerosis and frontotemporal dementia. The molecule, small enough to cross the blood-brain barrier, binds to the RNA structures that cause these diseases and promotes their elimination.
Familial amyotrophic lateral sclerosis and frontotemporal dementia are caused by the expansion of a six-nucleotide repeat in intron 1 of the C9orf72 gene. While healthy individuals typically show fewer than 30 repeats, patients with these diseases show more than 65, often thousands. As a result of the expansion, once the gene is transcribed, the RNA in intron 1 becomes toxic to neurons through two mechanisms. On the one hand, when translated it gives rise to toxic proteins. On the other hand, the RNA sequesters RNA-binding proteins, compromising their availability in the cell, and forms toxic structures in the nucleus.
Given the absence of curative treatments for familial amyotrophic lateral sclerosis and frontotemporal dementia, the team of Scripps Institute researchers led by Matthew Disney considered how to tackle toxic RNA. One strategy for eliminating messenger RNA is to use complementary oligonucleotides that tag it for removal. However, since the RNA has repetitive sequences, this approach could not be used, so the researchers came up with another possibility: designing a small molecule that could bind to the toxic RNA and trigger its elimination.
Duchenne Muscular Dystrophy
Duchenne Muscular Dystrophy is another so-called rare disease. Duchenne muscular dystrophy (DMD) is the most common and deadly type of muscular dystrophy, characterized by progressive muscle degeneration and weakness due to alterations in a protein called “dystrophin” that helps keep muscle cells intact. The disease affects mostly boys, but in rare cases it can also affect girls.
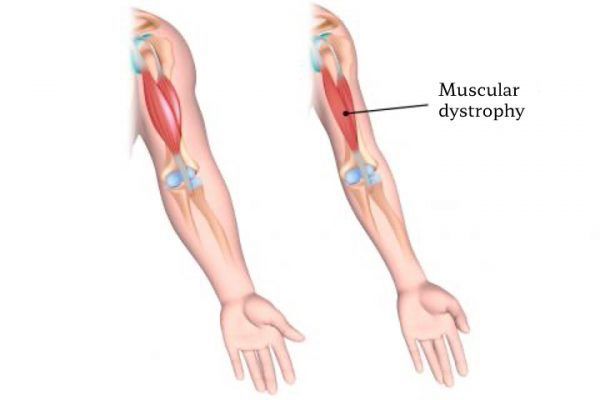
Researchers in India are working on the development of an affordable treatment for a rare and incurable genetic disorder called Duchenne muscular dystrophy, with more than 5,000 cases in the country.
According to scientists, muscle weakness is the main symptom of DMD. It can begin as early as 2 or 3 years of age, first affecting the proximal muscles (those close to the core of the body) and then affecting the distal limb muscles (those close to the limbs). Usually, the lower external muscles are affected before the upper external muscles. The affected child may have difficulty jumping, running and walking.
Other symptoms include enlargement of the calves, waddling gait and lumbar lordosis (an inward curvature of the spine). Later, the cardiac and respiratory muscles are also affected. Progressive weakness and scoliosis result in impaired lung function, which can eventually cause acute respiratory failure.
Researchers are working on affordable therapies for DMD and improving the efficacy of antisense oligonucleotide (ASOs)-based therapies.
According to Arun Shastry, scientific director of DART (Dystrophy Annihilation Research Trust), Bangalore, the idea of ASO-based therapy is to hide or mask specific exons (a segment of a DNA or RNA molecule that contains information encoding a protein) in a genetic sequence.
Oligos against neurodegenerative diseases
And it is not the only good news, because a team of Spanish researchers has developed a new patented technology with lipid nanoparticles could address the treatment of brain pathologies related to inflammatory or neurodegenerative processes, as reported in a collaborative work of the groups of CIBER de Salud Mental (CIBERSAM) at the Complutense University of Madrid, Hospital 12 de Octubre, CSIC and IDIBAPS; which has been published in Frontiers in Molecular Biosciences.

Antisense oligonucleotides (ASOs) are powerful gene therapy tools in development to address these pathologies, but there are limitations to their use. The main challenges are to avoid their degradation in peripheral tissues, such as blood, and to facilitate access to the brain in appropriate quantities to control gene expression in target cells. This new Spanish advance in delivery systems may overcome some of these barriers in some promising drugs.
From OLIGOFASTX we continue working to contribute with our grain of sand to alleviate the effects of some of these rare diseases, with the framework of scientific collaboration widely open. Only the joint work of the community will be able to provide solid research results to offer effective therapies. We will soon report further advances in our research. In the meantime, we will follow the news.

 Español
Español
