In recent years, the field of biomedicine has witnessed groundbreaking advances in the development of innovative therapies for a wide range of diseases
Among these innovations, the development of oligonucleotide therapies has experienced a great development and in particular the field of antisense therapies with paradigmatic examples such as the approval of several drugs against rare diseases such as hereditary amyloidosis, acute hepatic porphyria, or Duchenne muscular dystrophy. A concrete example of antisense therapy is the inhibition of the expression of microRNAs.
MicroRNAs (miRNAs) are small non-coding RNA molecules that play a crucial role in the regulation of gene expression. They act by binding to messenger RNA (mRNA) molecules and, depending on the context, they can inhibit protein translation or facilitate mRNA degradation. This regulatory capacity makes miRNAs essential for the maintenance of diverse cellular functions. However, when the levels of certain miRNAs are deregulated, they can contribute to the development of diseases such as cancer, cardiovascular diseases, neurological disorders and viral diseases. On the other hand, given the large number of genes whose expression is regulated by a single miRNA, these are considered particularly relevant therapeutic targets in complex and multigenic diseases, since by regulating a single miRNA it is possible to affect a specific pathway in several parts of the same pathway, thus achieving a theoretically more potent effect than modulating a single gene.
Anti-miR drugs are designed to inhibit the activity of specific miRNAs that are deregulated in diseases. These treatments use synthetic molecules, namely antisense oligonucleotides, which bind to the target miRNAs and prevent their interaction with the mRNAs. This allows normal gene regulation to be restored and offers a novel and promising therapeutic strategy. It is worth noting that although several companies have been working for years to develop drugs that inhibit the expression of microRNAs, and several of them have reached clinical development, none of them have yet been approved. As representative examples of the sector, Regulus Therapeutics in the US has specialised in the development of antimiRs against liver diseases, Cardior in Germany is focused on heart diseases, Resalis Therapeutics is developing antimiRs to treat metabolic diseases, and Arthex Biotech in Spain is developing antimiRs against neuromuscular diseases.
Preclinical development of antimiRs
The development of antimiRs (at Arthex) follows a rigorous optimisation and validation process to ensure the selection of the most effective and safe compounds to treat a disease. This process can be applied to any antimiR or pathology, and consists of the following steps.
To begin with, extensive research is carried out to understand the pathology of the target disease. This includes identifying the genetic and molecular causes that contribute to the disease and determining the proteins or microRNAs involved. A specific microRNA is then identified that is altered in patients and plays a key role in the pathology. This microRNA becomes the therapeutic target for antimiR development.
The next step is the development of oligonucleotides, where a library of antimiRs that can inhibit the target microRNA is designed and synthesised. These antimiRs are chemically modified oligonucleotides to improve their stability and efficacy. In vitro evaluation involves transfection of the antimiRs into disease-relevant human cells, using different concentrations to assess their effect. Activity (EC50 and Emax) and toxicity (TC50) values are determined to calculate the therapeutic index, which helps to identify the most promising antimiRs. In addition, a dual screening strategy is used to evaluate combinations of chemical modifications and hydrophobic conjugates for improved cell entry to measure the biological activity and potential cytotoxicity of antimiRs. This type of screening allows the identification of the most effective and least toxic antimiRs.
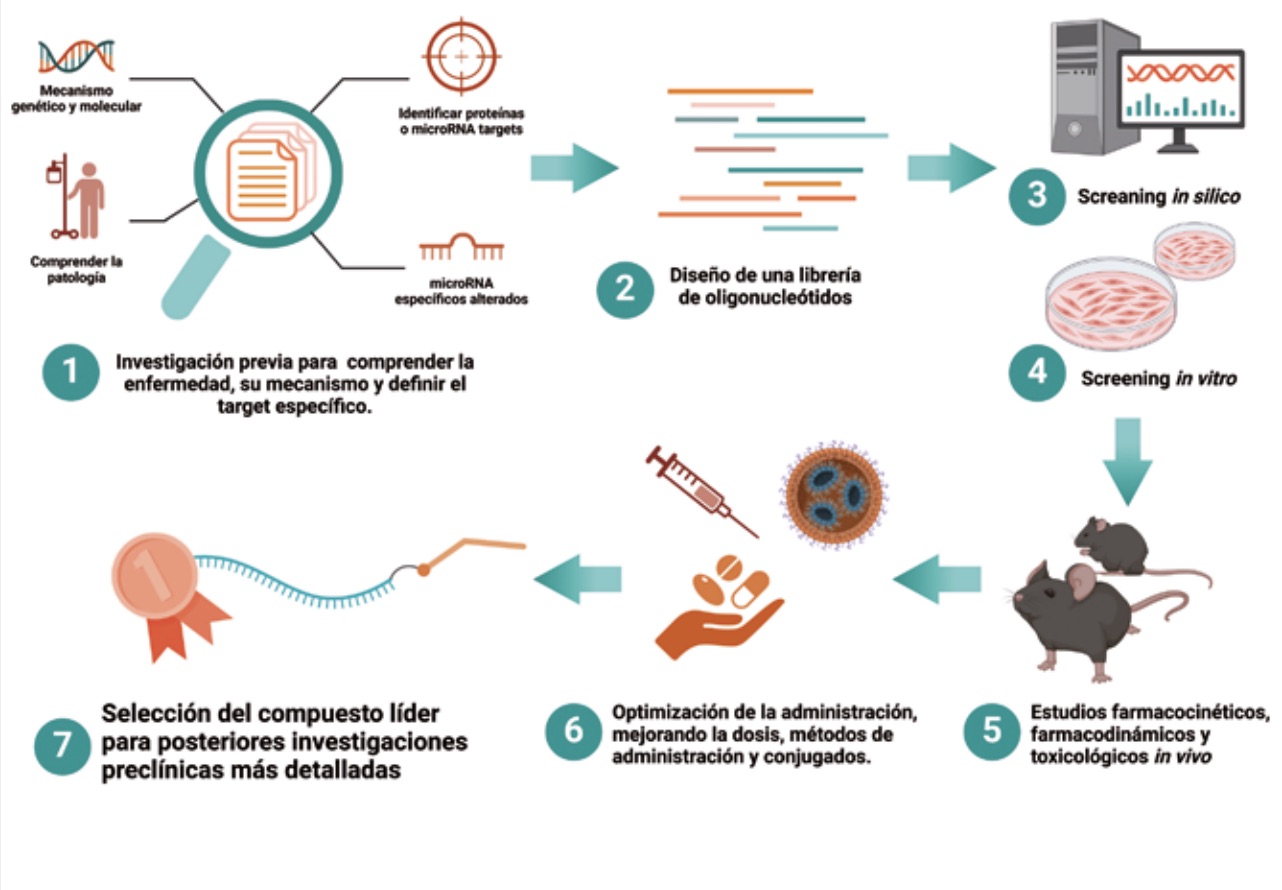
These selected antimiRs are then administered in animal models of the disease to assess their therapeutic impact. The impact of antimiRs on key disease biomarkers, such as specific protein levels, clinical phenotypes or even associated molecular events, is measured. In addition, ELISA and other methods are used to determine the distribution and quantity of antimiRs in different tissues, assessing their arrival in relevant tissues and their pharmacotoxic profile.
Finally, optimisation of administration includes testing different doses, timing and routes of administration to identify the most effective way to administer antimiRs. The efficacy of different antimiRs is compared, focusing on those with the best biodistribution profiles and durability of effects. Finally, the lead compound is identified based on its efficacy, safety and pharmacological profile. This allows the selection of an antimiR to advance to preclinical testing required by regulatory agencies before initiating a clinical trial.
At the regulatory level, antimiR, like other oligonucleotides, do not have a defined guideline from regulatory agencies such as the FDA or EMA. Generally, the guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are followed, namely ICH M3(R2) and ICH S6(R1). The former is focused on small molecules (or chemically synthesised molecules) while the latter is for biological molecules. AntimiRs are chemically synthesised molecules but their mechanism of action is similar to that of a biologic, hence the complexity in preclinical regulatory development. Special features include the selection of relevant species for preclinical studies, because the mechanism of action must be conserved in the target species (humans) and in the animal species to be tested.
Another important feature is the study of off-target effects, which although not indicated in the guidelines, will be required by regulatory agencies to assess and confirm that there will not be an undesirable effect due to interaction of the antimiR with a target other than the primary target. The regulatory package is completed by pharmacokinetic studies and in vitro and in vivo toxicity studies where both toxicity in the body after administration and the effect of the antimiR on the cardiovascular, respiratory and nervous system will be assessed. These studies allow safe doses to be established for the clinical trial in humans.
The pharmaceutical industry’s strong interest in the research and development of new oligonucleotide-based therapies is also reflected in the field of industrial property. Recent years have seen an exponential increase in the number of published patent applications related to oligonucleotides, as can be seen in the Espacenet database (worldwide.espacenet.com).
Thus, in general, it is possible to protect inventions related to therapeutic oligonucleotides, including antimiRs, by fulfilling the classic patentability criteria (novelty, inventive step and sufficiency of description). This includes having demonstrated the therapeutic effect of the antimiR in in vivo or in vitro models. However, there are certain nuances that, although introduced at the level of antimiRs, can be extrapolated to any oligonucleotide. Firstly, the scope of protection can be at the level of the antimiR itself, as a product, or by its therapeutic use. The strategy will depend on whether there is a known miRNA target for the disease for which the antimiR is being investigated or developed.
Generally, if the disease target miRNA is not previously known, therapeutic use of the antimiR can be claimed. In cases where the target miRNA is already known, protection is limited to the specific sequence of the antimiR and its chemical modifications, as designing an antimiR based on sequence complementarity is relatively simple.
In addition, in countries such as the US and Australia, chemical modifications must be included to signal the synthetic nature of the antimiR, which is essential for patentability. Patent offices, especially the European patent office, are becoming increasingly strict in these respects.
OLIGOFASTX. A comprehensive platform for the sustainable development of oligonucleotide-based therapies
OLIGOFASTX is a multidisciplinary consortium led by Sylentis (PharmaMar Group) with the participation of Arthex Biotech and four other Spanish companies, Nanovex Biotechnologies (Grupo Industrial Química del Nalón), AptaTargets, Nostrum Biodiscovery and 53Biologics.
OLIGOFASTX brings together resources and efforts to create a platform that facilitates and contributes to the generation in Spain of a biopharmaceutical industrial fabric specialised in the development of oligonucleotide-based therapies based on RNA, contributing to the accelerated development of this type of therapy. OLIGOFASTX is funded by the Centro de Desarrollo Técnico Industrial (CDTI) through the Misiones Ciencia e Innovación programme, within the Recovery, Transformation and Resilience Plan – Next Generation EU Funds.
Article for Farmabiotec written by:
Alba de Paz, Project Manager Arthex Biotech.
Estefanía Cerro, Head of R&D Arthex Biotech.
Nuria Barquero, Head of Non Clinical Development Arthex Biotech.

 Español
Español
